Address: No. 92, Xidazhi Street, Nangang District, Harbin



JOIN-USHIT sincerely invite talents from both home and aproad to join in the new course of constructing first-class universities. |
Recently, the research team led by Professor Bo Song from the Center for Composite Materials and Structures at the School of Astronautics, in collaboration with Professor Song Jin’s group at the University of Wisconsin-Madison, has made significant progress in the field of electrocatalytic water splitting. The research team innovatively employed hydrogen isotope labeling techniques combined with electrochemical analysis to precisely elucidate the microscopic kinetic mechanisms of proton transfer during water splitting, thereby providing a new theoretical tool for the design of efficient hydrogen-evolution catalysts. The related findings, entitled “Isotope-dependent Tafel analysis probes proton transfer kinetics during electrocatalytic water splitting”, were published online in the latest issue of Nature Chemistry.
Hydrogen energy is regarded as a key energy carrier for achieving carbon neutrality, yet the core bottleneck of electrocatalytic water splitting lies in its low reaction efficiency. For a long time, researchers have been unable to directly observe proton (hydrogen ion) transfer at the electrode-solution interface during water splitting, which has severely hindered further optimization of catalyst design.
To address this scientific challenge, the team substituted hydrogen atoms (H) in the reaction medium with deuterium (D), a stable isotope of twice the mass, and investigated proton transfer kinetics by tracking the catalytic performance variations arising from differences in isotopic zero-point energy. This isotopic substitution markedly reduces the vibrational frequency of O-H/O-D bonds, enabling precise detection of changes in the energy barrier for proton transfer across the electrode-solution interface.
The team integrated isotope effects with conventional electrochemical Tafel analysis: when reaction rate constants and activation barriers exhibit isotope-dependent differences, they established a new isotope-dependent Tafel correlation model, enabling precise determination of whether proton transfer is involved in the rate-determining step. This method overcomes the limitations of conventional kinetic isotope effect analyses, which are easily confounded by thermodynamic factors. It not only reflects evolutionary processes such as interfacial proton migration and surface adsorption processes, but also highlights the contribution of proton transfer steps to overall reaction kinetics, thereby distinguishing whether the rate-determining step is electrochemical or chemical in nature.
By testing six hydrogen-evolution catalysts and twenty-one oxygen-evolution catalysts, the researchers demonstrated the universality of this new isotope-Tafel correlation model in identifying rate-determining step characteristics. This study establishes a new evaluation standard and offers practical solutions for the design and performance optimization of next-generation high-efficiency electrolyzers.
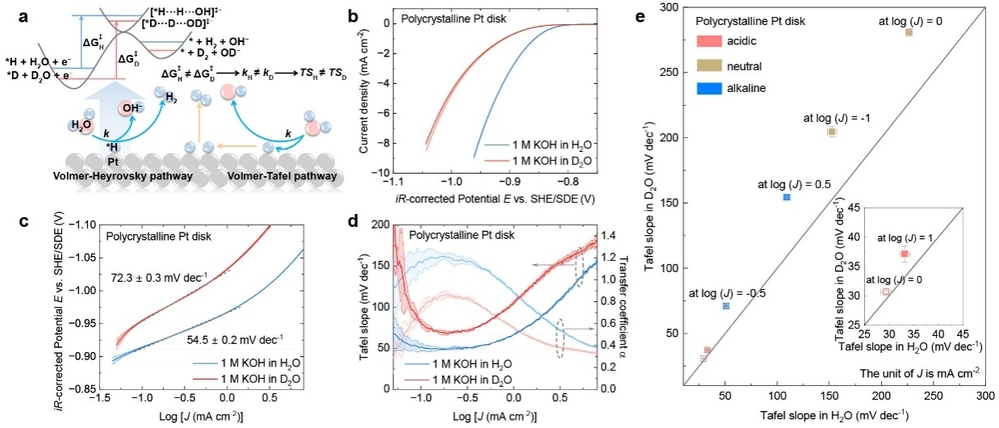
Relationship between hydrogen evolution kinetics and Tafel slope before and after H/D isotope substitution
Harbin Institute of Technology is the first and corresponding institution for this paper. Professor Bo Song, Professor Song Jin, and Dr. Hongyuan Sheng, a research fellow at Fudan University, are co-corresponding authors. Dr. Jinzhen Huang, a Ph.D. graduate from the School of Astronautics, is the first author. This research was supported by the National Science Foundation of China, the Frontiers Science Center Program of the Ministry of Education, the Heilongjiang Touyan Innovation Team Program, and institutional research projects of the Zhengzhou Research Institute of Harbin Institute of Technology.